PPESTANDARDS
When buying industrial gloves, an understanding of the numeric or alphabetical codes and symbols used for specifying protection levels in the standards is recommended.
CE CATEGORY
European Directive 89/686/EEC

CATEGORY I – Minor risks.
CATEGORY II – Reversible risks (injury), certified compliant by a notified body.
CATEGORY III – Irreversible risks (corrosion), certified compliant and tested by a notified body whose number is specified.
EN 420
General Requirements for Protective Gloves
- Technical information*
- Glove markings
- Sizes
- Level of dexterity (1 to 5)
- Innocuousness of the glove
EN ISO 374:2016
Gloves Giving Protection From Chemicals and Microorganisms
The standard defines the capability of gloves to protect the user against penetration, permeation and degradation by chemicals and microorganisms. It classifies three types of gloves by level of protection (A, B, and C).
EN 374-2:2014
Penetration Resistance
| Performance level | Acceptable quality level unit | Inspection levels |
| Level 3 | under 0,65 | G1 |
| Level 2 | under 1,5 | G1 |
| Level 1 | under 4,0 | S4 |
EN 1149-1
Antistatic properties
Tested level of glove surface resistivity. Measured in ohms/square (Ω), this indicates the capacity of the glove to disperse via a dissipative and/or conductive effect the accumulated static electricity discharges on the operator’s hand.
RISK RELATED TO FOOD CONTACT
It is applied to materials and articles that, at finished state, are intended to come into contact or are brought into contact with foodstuffs or with water that is for human consumption. According to Regulation 1935/2004: «The materials and articles must be manufactured in accordance with good manufacturing practice so that, under normal or foreseeable conditions for their use, they do not transfer their constituents to food in quantities which could:
- Present a danger to human health,
- Results in an unacceptable change in the composition of the foodstuffs or a deterioration in the organoleptic characteristics thereof.
All SHOWA gloves with the «food contact» logo are conform to Regulation (EU) No 1935/2004 and the Regulation (EU) No 2023/2006.
EN 16523-1: 2015
(replaces EN 374-3) Resistance to chemical permeation – Until 21/04/2018
| Measured breakthrough time | Permeation performance index |
| >10 | 1 |
| >30 | 2 |
| >60 | 3 |
| >120 | 4 |
| >240 | 5 |
| >480 | 6 |
| Type of gloves | Breakthrough time |
| A | ≥ 30 min for at least 6 chemicals |
| B | ≥ 30 min for at least 3 chemicals |
| C | ≥ 10 min for at least 1 chemicals |
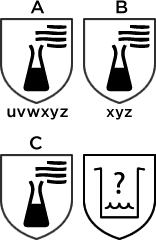
| Letter code | Chemical | CAS number | Class |
| A | Methanol | 67-56-1 | Primary alcohol |
| B | Acetone | 6764-1 | ketone |
| C | Acetonitrile | 75-05-8 | Nitrile compound |
| D | Dichloromethane | 75-09-2 | Chlorinated hydrocarbon |
| E | Carbon disulphide | 75-15-0 | Organic compound containing sulphur |
| F | Toluene | 7108-88-3 | Aromatic hydrocarbon |
| G | Diethylamine | 109-89-7 | Amine |
| H | Tetrahydrofurane | 141-78-6 | Heterocyclic ether |
| I | Ethyl acetate | 141-78-6 | Ester |
| J | n-Heptane | 1310-73-2 | Saturated hydrocarbon |
| K | Caustic soda 40% | 75-09-2 | Inorganic base |
| L | Sulphuric acid 96% | 7664-93-9 | Inorganic mineral acid |
| M* | Nitric acid 65% | 7697-37-2 | Inorganic mineral acid, oxidizing |
| N* | Acetic acid 99% | 64-19-7 | Organic acid |
| O* | Ammonium hydroxide 25% | 71336-21-6 | Organic base |
| P* | Hydrogen peroxide 30% | 7722-84-1 | Peroxide |
| S* | Hydrofluoric acid 40% | 7664-39-3 | Inorganic mineral acid, contact poison |
| T* | Formaldehyde 37% | 50-00-0 | Aldehyde |
EN 374-4: 2013
Resistance to chemical degradation
EN 374-5:2016
Protection against micro-organisms
EN 388:2016
Mechanical Risks
Revision of EN 388: 2003
The EN 388 standard underwent revision in 2016. SHOWA gloves are in the process of being recertified by the notified bodies to conform to the revised standard. Currently reported ISO 13997 cut resistance values are indications until officially certified. In the meantime the existing certificates according to EN 388: 2003 remain valid.
a) ABRASION RESISTANCE (0-4)
Number of cycles required to abrade a hole using abrasive paper in a circular sample of glove material under constant pressure and motion.
b) BLADE CUT RESISTANCE BY COUP TEST (0-5)
Number of cycles required to cut a sample using a stainless steel circular blade under constant speed and low force of 5 newtons (approx. 510g). For materials that dull the blade, after a certain number of cycles without cut through, the ISO 13997 test is performed and becomes the reference cut resistance value.
c) TEAR RESISTANCE (0-4)
Force required to propagate a tear in a rectangular sample of a glove with a starting incision, to a maximum force of 75N (approx. 7,6kg).
d) PUNCTURE RESISTANCE (0-4)
Force required to puncture the sample with a standard size steel point at a constant speed of 10 cm/min.
e) BLADE CUT RESISTANCE BY ISO TEST (A-F)
Force in newtons (N) required to cut through a sample using a rectangular blade in a specified cut test machine such as Tomodynamometer (TDM). This test is optional unless the blade in Coup test becomes dull, whereupon it becomes the reference for cut resistance. A letter value is assigned as follows:
Level of protection | A | B | C | D | E | F |
| Force in newtons | >2 | ≥5 | ≥10 | ≥15 | ≥22 | ≥30 |
| Force in newtons | Low | Medium | Medium | High | High | High |
f) IMPACT RESISTANCE (P) For protective gloves claiming impact resistance. Measures dissipation of force by the area of protection upon an impact of a domed anvil at an impact energy of 5 joules. Testing is carried out in accordance with the impact protection test for motorcycle protective gloves of EN 13594:2015 standard. A letter “P” is added on successful pass, while a fail remains unmarked.
Level X can also be applied for a – f above, which means “not tested”.
| Level of protection | 1 | 2 | 3 | 4 | 5 |
| Abrasion resistance (number of cycles) | >100 | ≥500 | ≥2000 | ≥8000 | – |
| Blade cut resistance by Coup test (index) | > 1,2 | > 2,5 | ≥ 5 | ≥ 10 | ≥ 20 |
| Tear resistance (force in newtons) | >10 | ≥25 | ≥50 | ≥75 | – |
| Puncture resistance (force in newtons) | >20 | ≥60 | ≥100 | ≥150 | – |
EN 511:2011
Cold-related risks
This standard applies to any gloves to protect the hands against convective and contact cold down to -50ºC.
Tested levels of glove performance in terms of the following risks:
- Climatic or industrial cold transmitted by convection (0 to 4).
- Climatic or industrial cold transmitted by contact (0 to 4).
- Impermeability to water (0 or 1).
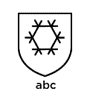
If the glove shows this symbol, it has achieved a performance index for (from left to right) climatic cold or industrial cold transmitted by convection, climatic cold or industrial cold transmitted by contact, impermeability to water.
“0” means that during the test level 1 was not reached.
“X” means that the test was not performed or not possible.
EN 407: 2011
Heat-related risks
Tested levels of glove performance in terms of the following risks:
- Resistance to flammability (0 to 4)
- Resistance to contact heat (0 to 4)
- Resistance to convective heat (0 to 3)
- Resistance to radiant heat (0 to 4)
- Resistance to small splashes of molten metal (0 or 1)
- Resistance to large splashes of molten metal (0 or 1)
“0” means that during the test level 1 was not reached. “X” means that the test was not performed or not possible.
EUROPEAN DIRECTIVE 93/42/EEC Covering medical examination and surgical gloves
EN 455-1
A random sample of gloves is tested for freedom of holes by undergoing a water leak penetration test. The gloves are filled with 1l of water and must remain completely leak proof over a defined period of time. A failed test results in a higher AQL value, which for medical gloves sold in Europe must be 1,5 or lower.
AQL (accepted quality level) is a quality sampling procedure ISO 2859-1 used by manufacturers for measuring the % likelihood of pinhole defects in a batch of single use gloves. An AQL of 1,5 brings a statistical probability that less than 1,5% of the gloves in the batch will have defects.
EN 455-2
Size and tensile strength requirements for single use medical gloves. No less than 240mm in median length and 95mm (±10mm) median width to provide adequate protection along full length of the hand (exception for long cuff gloves).
Strength is measured by elongation until breaking point, indicated as Force At Break (FAB) in newtons (N). FAB is measured on standard sample and on a rapid aged sample that is kept at 70°C for 7 days to simulate glove deterioration during prolonged shelf life. FAB requirements differ per glove material and if the glove is for examination or surgical purpose. Indication of median minimum FAB values:
Force at break (N) during shelf life
| Rubbers (e.g. natural latex, nitrile) | Thermoplastics (e.g. PVC, vinyl, butyl) | |
| Examination glove | ≥ 6,0 | ≥ 3,6 |
| Surgical glove | ≥ 9,0 | – |
EN 455-3
A number of important requirements are specified to maintain biological safety of the glove for the medical practitioner as well as the patient. “LATEX” pictogram on packaging for natural latex rubber gloves is mandatory. No terms suggesting relative safety of usage are permitted i.e. low allergenicity, hypoallergenicity or low protein content. Powder residue, which is seen as unwanted contaminant on medical gloves, must not exceed 2mg per glove with “powder-free” claim. Water extractable latex protein content in latex gloves must not exceed 50 microgram per gram of rubber to minimize latex exposure that can cause allergic reactions. The level of endotoxins generated by bacteria on sterile gloves that claim “low endotoxin level” may not exceed 20 EU per glove pair (EU=Endotoxin Units).